K6Sn4F12I2•0.5H2O: a zero-dimensional alkali metal tin mixed halide compound exhibiting color change due to crystal water loss
Abstract
A new zero-dimensional alkali-metal tin mixed halide, K6Sn4F12I2•0.5H2O, is synthesized by a hydrothermal method. It crystallizes in the cubic centrosymmetric space group of Fd-3m (No. 227) and its structure consists of crystal water molecules and ordered arranged [Sn4F12I4] fundamental structural blocks trapped in [K18] cages. Interestingly, K6Sn4F12I2•0.5H2O exhibits a color change from colorless to orange when exposed to air. Experimental measurements combined with theoretical calculations reveal that the color change in K6Sn4F12I2•0.5H2O is attributed to the loss of crystal water.
Keywords
INTRODUCTION
Metal halides have been researched in detail as a result of their structural and functional diversity, which has resulted in many important and interesting optoelectronic applications, such as photovoltaics, photoelectricity, nonlinear optics and photoluminescence[1-11]. According to the structure-property relationship, these optoelectronic properties are determined by their microstructures. For example, by reducing the structural dimensions from three dimensions to zero dimensions to promote the recombination of photogenerated carriers, (TTA)2SbCl5 and (bmpy)9[ZnCl4]2[Pb3Cl11] have realized ultrahigh photoluminescence quantum yield[12,13]. Moreover, through halogen element hybridization, metal halides, such as Cs2HgI2Cl2, Rb2CdBr2I2 and K2SbF2Cl3, [NH2(CH2CH3)2]3Bi(Cl1-xBrx)6, have significantly improved the laser damaged threshold and can maintain their nonlinear optical effects simultaneously, which make them good candidates as nonlinear optical materials in the infrared spectral region[14-17]. Considering their excellent optoelectronic properties and important applications, it is necessary to continue the research of structure-property relationships in metal halides.
Metal halides are normally obtained by solution or hydrothermal methods, where water can play an important role in determining their structures and optoelectronic properties. For example, by introducing water molecules to BiF3, Liu et al.[18] successfully regulated the optical birefringence by changing the coordination environment of Bi3+. Yang et al.[19] changed the luminescence color from orange to blue by recrystallizing Cs2InCl5 in water. Cordero et al.[20] investigated the roles of interstitial water in the structural transitions and stability of FAPbI3 and MaPbI3. Therefore, further investigation into the role of water molecules in the modulation of metal halide properties is essential.
Our group has focused on materials explorations of metal halides[21-24]. In our previous work, we reported dozens of metal halides with nonlinear optical effects, photodegradation effects and photoluminescence properties[25,26]. By introducing water, we successfully promoted the generation of more local photoelectrons and increased the photoluminescence quantum yield in metal halides[27]. In this work, a new zero-dimensional alkali-metal tin halide, K6Sn4F12I2•0.5H2O, is synthesized by a hydrothermal method. Its structure consists of crystal water molecules and fundamental structural blocks of [Sn4F12I4] trapped in [K18] cages. When exposed to air, its color slowly changes from colorless to orange. Experimental measurements combined with theoretical calculations reveal that it is crystal water loss that leads to the color change.
MATERIALS AND METHODS
Synthesis of K6Sn4F12I2•0.5H2O
K6Sn4F12I2•0.5H2O single crystals were synthesized by a hydrothermal method at a medium temperature of 493 K. The experimental reagents included NH4F, KI and SnCl2•2H2O, all of which were of analytical grade from commercial sources and used without further purification. A mixture of 1 g (0.027 mol) of NH4F, 1 g (0.006 mol) of KI and 1 g (0.004 mol) of SnCl2•2H2O was placed into the inner liner of a hydrothermal kettle and then 4 mL of deionized water were added. The hydrothermal kettle was sealed and placed in an oven. The mixture was heated to 493 K for 48 h and then cooled to room temperature with a rate of 3 °C/h. After that, colorless transparent K6Sn4F12I2•0.5H2O crystals were obtained.
Single-crystal structure determination
A small K6Sn4F12I2•0.5H2O crystal with dimensions of 0.12 × 0.10 × 0.08 mm3 was selected for single-crystal structure determination. The diffraction data were collected on a Rigaku AFC10 single-crystal diffractometer equipped with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) at 293 K. The crystal structures were solved by direct methods with the program SHELXS-97 and refined by full-matrix least squares on F2 by SHELXTL. The structures were verified using the ADDSYM algorithm from the program PLATON and no higher symmetry was found. The relevant crystallographic data, atomic coordinates, isotropic and anisotropic displacement parameters and selected bond distances and angles are listed in Supplementary Tables 1-4.
Powder X-ray diffraction
Powder X-ray diffraction (XRD) of the polycrystalline samples was performed at room temperature using an automated Bruker D8 Focus X-ray diffractometer equipped with a diffracted monochromator set for Cu Kα (λ = 1.5418 Å) radiation. A scanning step width of 0.02° and a scanning rate of 0.1°s-1 were applied to record the patterns in the 2Θ range of 10-70°.
Thermal analysis
Thermogravimetric analysis was performed on an EXSTAR TG/DTG thermogravimetric analyzer. The sample was placed in an alumina crucible, which was heated at a rate of 15 °C/min under N2 flow from room temperature to 900 °C.
UV transmittance spectroscopy
The UV transmittance spectra of K6Sn4F12I2•0.5H2O before and after the phase transition were collected with a Varian Cary 5000 UV-vis-NIR spectrometer in the wavelength range from 300 to 1400 nm. A BaSO4 plate was used as a standard.
Computational methods
The first-principles calculations for K6Sn4F12I2•0.5H2O were performed using the planewave pseudopotential method implemented in the CASTEP package based on density functional theory. The CA-PZ functionals of local density approximation were adopted to describe the exchange-correlation functionals. The planewave energy cutoff, self-consistent-field tolerance and k-point separation were set as 450 eV,
RESULTS AND DISCUSSION
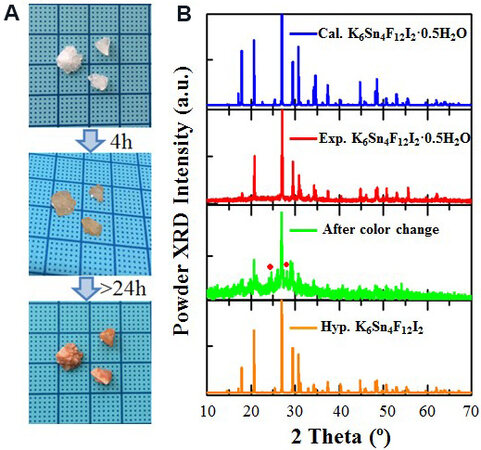
Single crystals of K6Sn4F12I2•0.5H2O were obtained by a hydrothermal method using the raw materials of 1 g (0.027 mol) of NH4F, 1 g (0.006 mol) of KI and 1 g (0.004 mol) of SnCl2•2H2O dissolved in 4 mL of deionized water. The mixture was reacted at 493 K and further slowly cooled to room temperature. Colorless transparent crystals of K6Sn4F12I2•0.5H2O were obtained [Figure 1A]. The crystal structure was solved and refined on the basis of single-crystal data. The powder XRD patterns of obtained crystals show good agreement with the calculated results derived from the single-crystal data [Figure 1B], indicating the purity of the obtained samples (crystal data, atomic coordinates, atomic displacement parameters and bond distances and angles are given in Supplementary Tables 1-4).
Figure 1. (A) Photographs of obtained single crystals with color change. (B) Powder XRD patterns of K6Sn4F12I2•0.5H2O. XRD: X-ray diffraction.
K6Sn4F12I2•0.5H2O crystallizes in the cubic centrosymmetric space group of Fd-3m (No. 227) with unit cell parameters of a = b = c = 17.1860 Å. All the atoms are occupied in one crystallographically unique position in the symmetric unit. The K, Sn, F, I, O and H ions are at Wyckoff positions 48f, 32e, 96g, 16c, 8b and 32e. In the structure, the Sn2+ cations are coordinated with three F- anions and form [SnF3] triangular pyramids for the presence of stereochemically active lone-pair electrons on Sn2+ cations. The Sn-F bonds length are
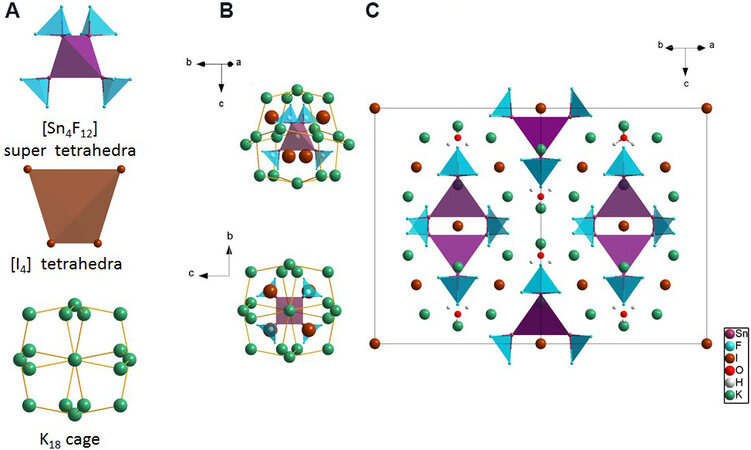
In order to illuminate the structure clearly, the [Sn4F12] supertetrahedra, [I4] tetrahedra and [K18] cage are displayed in Figure 2A. The [Sn4F12] supertetrahedra are composed of four [SnF3] triangular pyramids with the Sn2+ cations located at the vertex. Every four isolated I- anions can construct the [I4] tetrahedra, which are staggered with the [Sn4F12] supertetrahedra and formed the cubic [Sn4F12I4] fundamental structural blocks. In this cubic [Sn4F12I4] fundamental structural block, the [SnF3] triangular pyramids and I- anions occupy the eight vertexes, respectively. The K+ cations are inserted into the space between the [Sn4F12I4] fundamental structural blocks and every eighteen K+ cations construct a [K18] cage, as shown in Figure 2A. These [K18] cages further trap the cubic [Sn4F12I4] fundamental structural blocks, as shown in Figure 2B. The distance between the neighboring K+ cations in the [K18] cages is 4.4637 Å, which is large enough to hold the cubic [Sn4F12I4] fundamental structural blocks. All K+ cations are six-coordinated to F- anions with two short K-F bonds of 2.7735 Å and four long bonds of 2.8792 Å. The F-K-F angles vary from 57.632° to 123.107°, resulting in a planar arrangement of the four farther F- anions, which makes the [KF6] polyhedra adopt a saddle-like shape. These saddle-like polyhedra further construct the three-dimensional framework by sharing the edges, as shown in Supplementary Figure 1. After adding the crystal water molecules, the fundamental structural blocks construct the crystal structure of K6Sn4F12I2•0.5H2O shown in Figure 2C.
Figure 2. (A) [Sn4F12] supertetrahedra, [I4] tetrahedra and [K18] cage. (B) [Sn4F12I4] fundamental structural block in [K18] cage. Upper panel, view along the [210] direction, and lower panel, view along the a-axis. (C) Structure of K6Sn4F12I2•0.5H2O.
As shown in Figure 1A, when exposed to air, K6Sn4F12I2•0.5H2O exhibits a color change from colorless to orange within 24 h. It should be noted that this phenomenon only occurs on the surface, with the internal color unchanged, even after exposure in air for one month. As a result, a single-crystal structure solution is not available for the microscopic structure of the compound after the color change. In order to investigate the color change mechanism, experimental characterization of the powder samples (obtained by grinding the single crystals and exposing them to air for more than 24 h) and theoretical calculations are performed.
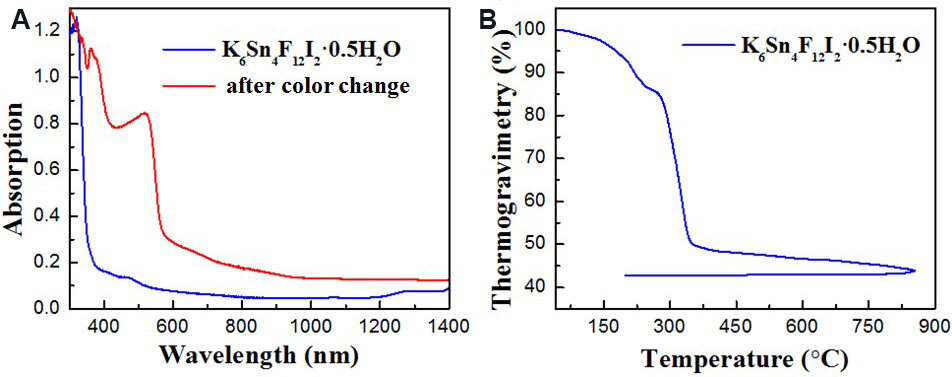
The powder XRD patterns of the samples after the color change are shown in Figure 1B. As shown, the patterns are highly consistent with those before the color change, except for the decrease in crystallinity and two peaks from unknown compounds. This means that the color change does not change the powder XRD patterns. UV-vis-NIR diffuse-reflectance spectra for the samples before and after the color change were measured and the results are exhibited in Figure 3A. As the results show, the shortest absorption edges are 350 and 565 nm for K6Sn4F12I2•0.5H2O before and after the color change, corresponding to 3.5 and 2.2 eV, respectively. The results also agree well with the sample colors before (colorless) and after (orange) the change. Thermogravimetric measurements were also carried out to evaluate the stability of
Figure 3. (A) UV-vis-NIR diffuse-reflectance spectra of K6Sn4F12I2•0.5H2O before (blue) and after (red) color change. (B) Thermogravimetric analysis curve of K6Sn4F12I2•0.5H2O.
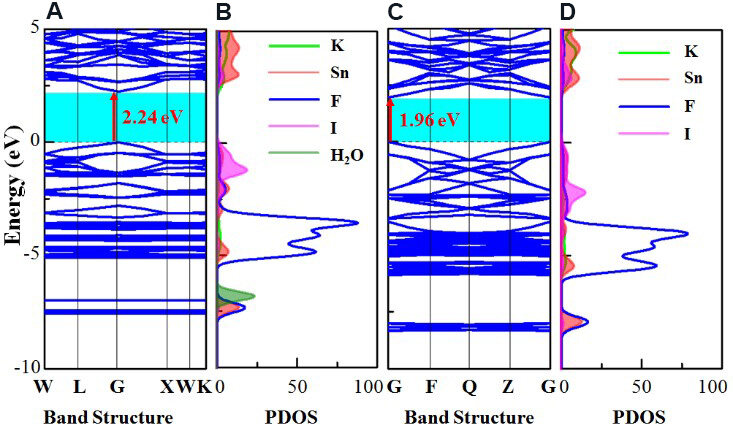
As the crystal structure after the color change is unavailable, first-principles calculations[30] on
CONCLUSIONS
In summary, a new alkali-metal tin halide, K6Sn4F12I2•0.5H2O, was synthesized by a hydrothermal method. It possesses a zero-dimensional structure consisting of crystal water molecules and [Sn4F12I4] fundamental structural blocks trapped in [K18] cages. Interestingly, K6Sn4F12I2•0.5H2O exhibits a color change from colorless to orange when exposed to air. Experimental measurements and theoretical calculations were performed and the results revealed that this color change is attributed to the loss of crystal water in the microstructure.
DECLARATIONS
AcknowledgmentsGong P acknowledge the support of Key Laboratory of Functional Crystals and Laser Technology, TIPC, CAS and Youth Innovation Promotion Association CAS.
Authors’ contributionsPerformed the experiments and calculations and wrote the initial draft of the manuscript: Gong P
Conceived the idea: Luo S
Provided the calculation package and analysis tools: Lin Z
All authors contributed to the general discussion.
Availability of data and materialsCrystallographical data (CIF) and additional data in Supplementary Materials. Deposition number CCDC 2104252 for K6Sn4F12I2•0.5H2O.
Financial support and sponsorshipThis work was supported by the National Natural Science Foundation of China Grants Nos. 11704023, 51802321, 51872297, and 51890864, and Chinese Academy of Sciences (Grants ZDRW-CN-2021-3).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
Supplementary Materials
REFERENCES
1. Zhang R, Mao X, Yang Y, et al. Air-stable, lead-free zero-dimensional mixed bismuth-antimony perovskite single crystals with ultra-broadband emission. Angew Chem Int Ed Engl 2019;58:2725-9.
2. Zhou L, Liao JF, Huang ZG, et al. A highly red-emissive lead-free indium-based perovskite single crystal for sensitive water detection. Angew Chem Int Ed Engl 2019;58:5277-81.
3. Zhou C, Lin H, Tian Y, et al. Luminescent zero-dimensional organic metal halide hybrids with near-unity quantum efficiency. Chem Sci 2018;9:586-93.
4. Saouma FO, Stoumpos CC, Wong J, Kanatzidis MG, Jang JI. Selective enhancement of optical nonlinearity in two-dimensional organic-inorganic lead iodide perovskites. Nat Commun 2017;8:742.
5. Hao F, Stoumpos CC, Cao DH, Chang RPH, Kanatzidis MG. Lead-free solid-state organic-inorganic halide perovskite solar cells. Nature Photon 2014;8:489-94.
6. Xing G, Mathews N, Sun S, et al. Long-range balanced electron- and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013;342:344-7.
7. Li L, Shang X, Wang S, et al. Bilayered hybrid perovskite ferroelectric with giant two-photon absorption. J Am Chem Soc 2018;140:6806-9.
8. You YM, Liao WQ, Zhao D, et al. An organic-inorganic perovskite ferroelectric with large piezoelectric response. Science 2017;357:306-9.
9. Connor BA, Leppert L, Smith MD, Neaton JB, Karunadasa HI. Layered halide double perovskites: dimensional reduction of
10. Chen B, Li T, Dong Q, et al. Large electrostrictive response in lead halide perovskites. Nat Mater 2018;17:1020-6.
11. Huang L, Wang Q, He F, et al. Synthesis, crystal structures and nonlinear optical properties of polymorphism: α- and β-RbHgI3·H2O. J Alloys Compd 2019;771:547-54.
12. Zhou C, Lin H, Neu J, et al. Green emitting single-crystalline bulk assembly of metal halide clusters with near-unity photoluminescence quantum efficiency. ACS Energy Lett 2019;4:1579-83.
13. Li Z, Li Y, Liang P, Zhou T, Wang L, Xie R. Dual-band luminescent lead-free antimony chloride halides with near-unity photoluminescence quantum efficiency. Chem Mater 2019;31:9363-71.
14. Wu Q, Meng X, Zhong C, Chen X, Qin J. Rb2CdBr2I2: a new IR nonlinear optical material with a large laser damage threshold. J Am Chem Soc 2014;136:5683-6.
15. Zhang G, Li Y, Jiang K, et al. A new mixed halide, Cs2HgI2Cl2: molecular engineering for a new nonlinear optical material in the infrared region. J Am Chem Soc 2012;134:14818-22.
16. Zheng X, Liu Y, Liu G, et al. Crystalline mixed halide halobismuthates and their induced second harmonic generation. Chem Mater 2016;28:4421-31.
17. Huang Y, Meng X, Gong P, Lin Z, Chen X, Qin J. A study on K2SbF2Cl3 as a new mid-IR nonlinear optical material: new synthesis and excellent properties. J Mater Chem C 2015;3:9588-93.
18. Liu X, Yang C, Wu Q, Sun J, Liang F. From BiF3 to BiF3·H2O: diverse Bi(iii) coordination for structural transformation and birefringence regulation. CrystEngComm 2020;22:6838-46.
19. Yang C, Guo F, Zhang Y, et al. Luminescence change from orange to blue for zero-dimensional Cs2InCl5(H2O) metal halides in water and a new post-doping method. Chem Asian J 2021;16:1619-25.
20. Cordero F, Craciun F, Paoletti AM, Zanotti G. Structural transitions and stability of FAPbI3 and MAPbI3: the role of interstitial water. Nanomaterials (Basel) 2021;11:1610.
21. Gong P, Luo S, Huang Q, et al. An alkaline tin(II) halide compound Na3Sn2F6Cl: synthesis, structure, and characterization. J Solid State Chem 2017;248:104-8.
22. Gong P, Luo S, Xiao K, et al. Structure and characterization of a zero-dimensional alkali tin dihalides compound Cs3Sn3F2Cl7 with the [Sn2F2Cl4]2- clusters. Inorg Chem 2017;56:3081-6.
23. Gong P, Yang Y, You F, et al. A SbF3Cl (A = Rb, Cs): structural evolution from centrosymmetry to noncentrosymmetry. Cryst Growth Des 2019;19:1874-9.
24. Song G, Li M, Yang Y, et al. Lead-free tin(IV)-based organic-inorganic metal halide hybrids with excellent stability and blue-broadband emission. J Phys Chem Lett 2020;11:1808-13.
25. Gong P, Luo S, Yang Y, et al. Nonlinear optical crystal Rb4Sn3Cl2Br8: synthesis, structure, and characterization. Cryst Growth Des 2018;18:380-5.
26. Song G, Li Z, Gong P, Xie R, Lin Z. Tunable white light emission in a zero-dimensional organic-inorganic metal halide hybrid with ultra-high color rendering index. Adv Optical Mater 2021;9:2002246.
27. Song G, Li M, Zhang S, et al. Enhancing photoluminescence quantum yield in 0D metal halides by introducing water molecules. Adv Funct Mater 2020;30:2002468.
28. Salami TO, Zavalij PY, Oliver SR. Synthesis and crystal structure of two tin fluoride materials: NaSnF3 (BING-12) and Sn3F3PO4. J Solid State Chem 2004;177:800-5.
29. Zemnukhova LA, Fedorishcheva GA. Study of isomorphism in fluorine-containing antimony(III) complexes. Russ Chem Bull 1999;48:104-9.
30. Clark SJ, Segall MD, Pickard CJ, et al. First principles methods using CASTEP. Z Kristallogr Cryst Mater 2005;220:567-70.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Gong P, Luo S, Lin Z. K6Sn4F12I2•0.5H2O: a zero-dimensional alkali metal tin mixed halide compound exhibiting color change due to crystal water loss. Microstructures 2022;2:2022004. http://dx.doi.org/10.20517/microstructures.2021.07
AMA Style
Gong P, Luo S, Lin Z. K6Sn4F12I2•0.5H2O: a zero-dimensional alkali metal tin mixed halide compound exhibiting color change due to crystal water loss. Microstructures. 2022; 2(1): 2022004. http://dx.doi.org/10.20517/microstructures.2021.07
Chicago/Turabian Style
Gong, Pifu, Siyang Luo, Zheshuai Lin. 2022. "K6Sn4F12I2•0.5H2O: a zero-dimensional alkali metal tin mixed halide compound exhibiting color change due to crystal water loss" Microstructures. 2, no.1: 2022004. http://dx.doi.org/10.20517/microstructures.2021.07
ACS Style
Gong, P.; Luo S.; Lin Z. K6Sn4F12I2•0.5H2O: a zero-dimensional alkali metal tin mixed halide compound exhibiting color change due to crystal water loss. Microstructures. 2022, 2, 2022004. http://dx.doi.org/10.20517/microstructures.2021.07
About This Article
Copyright
Data & Comments
Data

 Cite This Article 8 clicks
Cite This Article 8 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.