Triethanolamine assisted synthesis of bimetallic nickel cobalt nitride/nitrogen-doped carbon hollow nanoflowers for supercapacitor
Abstract
Supercapacitors (SCs) have drawn growing attention due to their advantages in fast charge/discharge over batteries. Benefiting from their prominent electrical conductivity and metal-like characteristics, transition metal nitrides have emerged as promising electrode materials for SCs. Traditional ways to prepare metal nitrides through ammonolysis are inconvenient and induce severe environmental pollution. Herein, we report a facile synthetic method toward heterogenous Ni3N-Co2N0.67/nitrogen-doped carbon (Ni3N-Co2N0.67/NC) hollow nanoflower via pyrolyzing NiCo-TEOA (triethanolamine) complex precursor applying urea as nitrogen source. Electrochemical tests demonstrate that the Ni3N-Co2N0.67/NC nanoflower delivers good specific capacitance (1582 F g-1 at 1 A g-1) and steady cycle performance (83.79% after 5000 cycles). Moreover, the as-assembled Ni3N-Co2N0.67/NC//AC cell can reach a peak energy density of 32.4 W h kg-1 at a power density of 851.3 W kg-1. The excellent electrochemical performance confirms extensive application prospects of the Ni3N-Co2N0.67/NC nanoflower.
Keywords
INTRODUCTION
The rapid development of clean and renewable energy in various fields including electric vehicles and portable electronic devices desires high-efficiency and high-capacity energy storage devices[1,2]. Supercapacitors (SCs) have attracted much interest due to their enhanced power density and long service life[3]. Standing in the intermediate zone of batteries and traditional capacitors, SCs could be generally divided into two types. Electric double-layer capacitors (EDLCs) rely on the electrostatic attraction of ions at the interfaces between electrode and electrolyte to complete charge storage, whereas pseudocapacitors (PCs) take advantage of redox reactions during faradaic redox processes to store electric energies[4,5]. Importantly, developing SCs with stronger energy storage capacity inevitably demands the utilization of better electrode materials[6].
Numerous transition metal nitrides (TMNs) such as Ni3N[7], Co2N[8], Fe2N[9], VN[10] and MoN[11] have emerged as potential electrode materials for SCs by virtue of their distinctive electronic structure, stable chemical resistance, remarkable electric conductivity, and flexible mechanical deformability[12]. However, most of the TMNs are synthesized by pyrolyzing the precursor under NH3 atmosphere, which leads to massive waste of NH3 and causes immeasurable environmental pollution. Consequently, there is a desperate need to develop a more convenient and green approach to prepare TMNs. Importantly, applying nontoxic and environmentally friendly nitrogen sources is a priority. Nitrogenous organic small molecules, which are easy to store and can produce NH3 under high temperatures, might serve as ideal substitutes for NH3. For example, Yang et al. converted vanadium-organic compounds (VAORCS) into vanadium nitride quantum dots/nitrogen-doped hierarchical carbon nanocomposites (VNQD/NDHCs) by annealing the mixture of VAORCS powder and melamine[13]. Jin et al. mixed chloride salts of five different metals with urea by ball-milling to form a metal-urea gel and obtained high-entropy metal nitride via calcining the gel[14]. Inspired by these previous reports, we chose urea as the nontoxic and cheap nitrogen source to prepare metal nitrides. In addition, different from individual ones, bimetallic nanoparticles often exhibit higher catalytic activities, richer redox sites, and better chemical stabilities[15-17]. Meanwhile, nickel and cobalt are chosen because they have comparable atomic size and chemical valence state[18].
On the other hand, it is known to all that the most important factor affecting the performance of materials is their morphology and structure. Compared to solid structures, hollow ones possess large inner voids, reactive inner surfaces and indestructible structures[19]. Hence, constructing hollow structures with low mass transport resistance, rapid ion diffusion channels and high-volume electrical capacity stands out as an efficient strategy to enhance SCs performance[20]. Metal-small organic molecule complexes are ideal precursors for hollow structures. Liu et al. coordinated Ni2+ and Co2+ with glycerol and then transformed the solid complex into a yolk-shell structure via the hydrothermal method[21]. Dong et al. synthesized hollow carbon spheres by etching SiO2 template with HF[22]. To avoid the use of a template and multifarious synthesis steps, we designed a one-step strategy toward hollow structure by coordinating Ni2+ and Co2+ with triethanolamine (TEOA) accompanied by the hydrolysis of metal alkoxide. Meanwhile, the nitrogenous organic network could be pyrolyzed into N-doped carbon via calcine, which further enhances the electrical conductivity and serves as strong support during long-term cycling[23].
Herein, we reported a hierarchical Ni3N-Co2N0.67/nitrogen-doped carbon (NC) hollow nanoflower, which is derived from annealing nickel/cobalt-TEOA complex (N1C2-TEOA) precursor with urea as nitrogen source. The as-prepared Ni3N-Co2N0.67/NC delivers larger specific surface area, superior energy storage capacity and longer cycle lifespan. The Ni3N-Co2N0.67/NC transformed from N1C2-TEOA sample shows an excellent specific capacitance of 1582 F g-1 at 1 A g-1 and 83.79% capacitance retention after 5000 cycles. Furthermore, the assembled Ni3N-Co2N0.67/NC//AC asymmetric device demonstrates a maximum energy density of
MATERIALS AND METHODS
Materials
Nickel chloride hexahydrate (NiCl2∙6H2O), Cobalt chloride hexahydrate (CoCl2∙6H2O), triethanolamine (TEOA) and urea were all purchased from Shanghai Aladdin Biochemical Technology Co., Ltd and used without further purification. The deionized water (DI water) involved in the experiment with an electrical resistivity of 18.2 MΩ cm-1 was prepared by ultrapure water polishing system.
Sample preparation and characterization
Synthesis of heterogenous Ni3N-Co2N0.67/NC hollow nanoflowers
Firstly, 2 g TEOA was dissolved in 18 mL DI water under vigorous stirring. Subsequently, x mmol
The heterogenous Ni3N-Co2N0.67/NC was synthesized by the following process. Initially, 50 mg N1C2-TEOA and 500 mg urea (mass ratio: 1:10) were uniformly dispersed in the porcelain boats. Then the boat with urea and N1C2-TEOA was placed upstream and downstream of the tube furnace, respectively. The furnace was heated to 400 °C at a heating rate of 2 °C min-1 under N2 atmosphere and kept for 2 h. After natural cooling down to room temperature, heterogenous Ni3N-Co2N0.67/NC hollow nanoflowers were obtained.
Structure and morphological characterization
X-ray diffraction (XRD) was tested using Rigaku Ultimate IV powder X-ray diffractometer with Cu Kα radiation (λ = 1.5418 Å) at a scanning speed of 5°/min. Scanning electron microscopy (SEM) was performed on Zeiss sigma 300 scanning electron microscope. Transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SAED) were carried out on FEI Talos F200x transmission electron microscope. Brunauer-Emmett-Teller (BET) specific surface areas and pore volumes were measured on ASAP 2460. X-ray photoelectron spectroscopy (XPS) data was collected on Thermo Scientific K-Alpha using Al Ka X-ray as the excitation source
Electrochemical characterization
The electrochemical measurements were tested by a three-electrode configuration in 1 M KOH electrolyte. Platinum electrode and saturated calomel electrode were used as the counter electrode and reference electrode, respectively. All the electrochemical performance was studied on a CHI760E electrochemical workstation. The working electrode was fabricated by the following procedures. Active material
where I, Δt, m, ΔV indicate the applied current (A), discharge time (s), the mass load of the active material (g) and the working potential (V), respectively.
In the Ni3N-Co2N0.67/NC//AC asymmetric supercapacitor system, active carbon (AC) was applied as the negative electrode material, which was prepared using the same steps as the positive electrode. The mass of AC can be computed based on the charge balance equation showing as follows[25]:
where m+, m-, C+, C-, V+, V- represent the mass (g), specific capacitance (F g-1) and operating voltage window (V) of the positive and negative electrode, respectively.
In addition, the energy density (Wh kg-1) and power density (W kg-1) at different current densities were calculated from the following Eqs.[26]:
where C, ΔV, Δt are the specific capacitance (F g-1), working potential (V) and discharge time (s) of the device, respectively.
RESULTS AND DISCUSSION
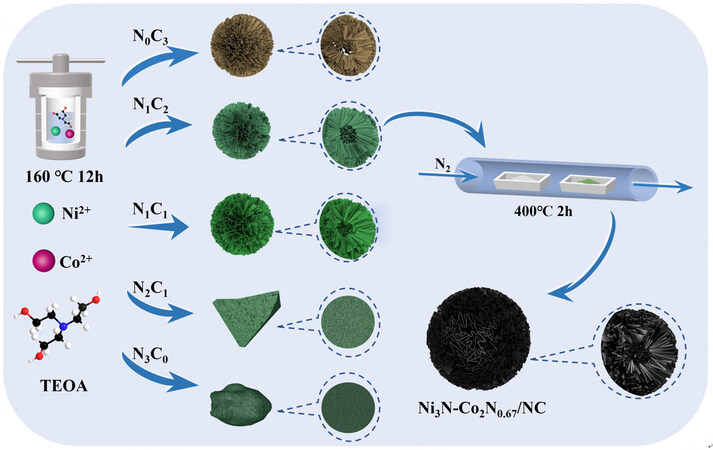
As schemed in Figure 1, the complete synthesis route of Ni3N-Co2N0.67/NC includes two steps. In the first hydrothermal process, TEOA could serve as an ideal solvent and more importantly as a ligand for metal complexes[27]. To avoid inhibition of the complex growth kinetics and nonuniform dispersion of the reaction system due to strong viscosity of TEOA, DI water was chosen as the only cosolvent. Initially, TEOA molecules react with Ni (II) and Co (II) to form NixCoy-TEOA complex at low temperatures. With the temperature rising, this metal alkoxide begins to hydrolyze, leading to the hollow porous nanoflower structure[21]. In the subsequent thermal treatment, carbon skeleton is pyrolyzed at 400 °C and transformed to N-doped carbon owing to the existence of N center atom of TEOA. Simultaneously, urea decomposes into NH3 which further reacts with Ni (II) and Co (II), producing Ni3N and Co2N0.67 eventually. Additionally, various mass ratios of Ni2+ to Co2+ (0:3, 1:2, 1:1, 2:1, 3:0) were used to investigate the impact on morphology and electrochemical performance of the complex and the best one was chosen to further derivative to the final product.
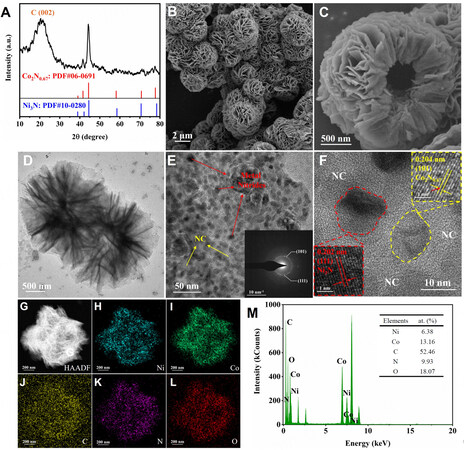
XRD patterns of N0C3-TEOA, N1C2-TEOA, N1C1-TEOA, N2C1-TEOA and N3C0-TEOA are presented in Supplementary Figure 1. As expected, all the precursors show no sharp and distinct peaks, exhibiting salient amorphous characteristics[28]. The wide peak bulge at 15°-25° is assigned to the (002) crystal face of carbon, indicating the successful formation of nitrogen-doped carbon after calcining [Figure 2A][29]. The peaks at 38.9°, 42.1°, 44.5°, 58.5°, 70.6° and 78.4° could be attributed to the (110), (002), (111), (112), (300) and (113) crystal faces of Ni3N (JCPDS:06-0691), while the peaks at 39.0°, 41.6°, 44.4°, 58.2°, 70.7° and 77.7° could correspond to the (100), (002), (101), (102), (110) and (103) crystal faces of Co2N0.67 (JCPDS:10-0280). All these peaks are sharp and narrow due to the good crystallinity of the material[30]. Moreover, no peaks were observed for nickel oxide or cobalt oxide, suggesting pristine metal nitrides were obtained.
Figure 2. (A) XRD pattern of Ni3N-Co2N0.67/NC. (B and C) SEM images of Ni3N-Co2N0.67/NC. (D) TEM image. (E and F) high-resolution TEM image (Inset: SAED image). (G) high-angle annular dark field TEM image. (H and L) the EDS mapping images of Ni, Co, C, N, and O elements. (M) EDS diagram. XRD: X-ray diffraction; SEM: scanning electron microscopy; TEM: transmission electron microscopy; SAED: selected area electron diffraction.
The morphologies of precursors were investigated by SEM as displayed in Supplementary Figure 2A-J.
TEM was employed to gain a better understanding of the internal microstructure of Ni3N-Co2N0.67/NC. As can be seen in Figure 2D, Ni3N-Co2N0.67/NC shows a typical nanoflower structure with a hollow inner core, which is well consistent with SEM images. HRTEM was then carried out to analyze the composition of
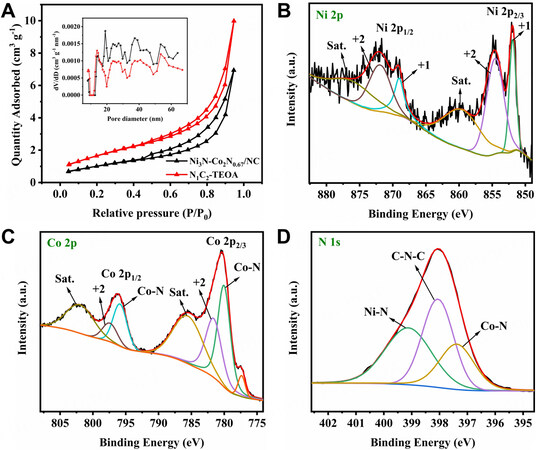
To investigate the pore nature of the precursors and Ni3N-Co2N0.67/NC, N2 adsorption-desorption measurement was conducted, as shown in Supplementary Figure 3A-D and Figure 3A. All the isotherms belong to type IV, indicating their mesoporous characteristics[32]. The specific surface areas of N0C3-TEOA, N1C2-TEOA, N1C1-TEOA, N2C1-TEOA and N3C0-TEOA are found to be 18.88 m2g-1, 20.98 m2g-1,
Figure 3. (A) N2 adsorption-desorption isotherms of Ni3N-Co2N0.67/NC and N1C2-TEOA (Inset: pore size distribution pattern). High-resolution XPS spectrum and simulation of (B) Ni 2p, (C) Co 2p and (D) N 1s for Ni3N-Co2N0.67/NC.
The changes in chemical bonds are characterized by FTIR exhibited in Supplementary Figure 4. The peaks at around 3400 cm-1 are ascribed to the -OH group of adsorbed water molecules[33]. For the precursors, the peaks at about 2842-3062 cm-1 are related to the stretching vibration of -CH2-, while the peaks at
XPS was determined to observe the surficial element and electronic interaction. Supplementary Figure 5 demonstrates the survey spectra of Ni3N-Co2N0.67/NC, evidencing the presence of Ni, Co, C, N and O elements. For the Ni 2p spectra in Figure 3B, the peaks located at 854.57 and 871.94 eV are attributed to oxidized Ni2+, while the peaks centered at 851.92 and 869.04 eV can be indexed to Ni+ species of Ni-N bonds[37]. For the Co 2p spectra in Figure 3C, the peaks at 781.62 and 797.41 eV are assigned to Co2+ and the peaks at 780.03 and 795.86 eV are caused by Co-N bonds[38]. The results above reveal the existence of Ni3N and Co2N0.67, which is further proved in N 1 s spectra in Figure 3D. The peaks at 399.12 and 397.38 eV belong to Ni-N and Co-N bonds, respectively[39,40]. Moreover, the peak at 398.06 corresponds to C-N-C groups, suggesting the successful establishment of nitrogen-doped carbon network[41]. Nitrogen doping could enhance the electrical conductivity of carbon, thus improving the energy storage performance of
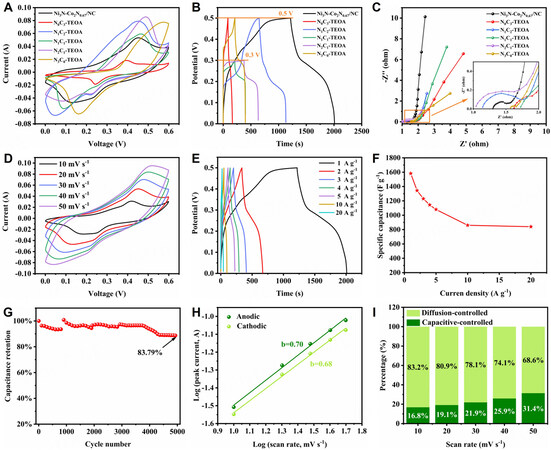
The electrochemical performances of all the samples were conducted in a three-electrode system in 1 M KOH aqueous electrolyte. As plotted in Figure 4A, the CV curves of all the samples at 20 mV s-1 show distinct redox peaks, revealing their energy storage mechanism of the combination of pseudocapacitance and double-layer capacitance[43]. To choose the best precursor for further nitridation, we initially compared the specific capacitance of all the precursors. The galvanostatic charge-discharge (GCD) profiles at 1 A g-1 displayed in Figure 4B of all the precursors show evident charge-discharge plateaus, which is well consistent with CV result. Interestingly, N1C1-TEOA and N2C1-TEOA could only reach a working potential window of 0.3 V with capacitances of 647 and 873 F g-1, respectively. N0C3-TEOA, N1C2-TEOA and N3C0-TEOA could reach a working potential window of 0.5 V with capacitances of 158, 972 and 382 F g-1, respectively. Obviously, N1C2-TEOA exhibited the best capacitive performance among the precursors and owned a favorable structure, so it was chosen for further annealing. As expected, Ni3N-Co2N0.67/NC possessed the longest discharge time and an operating potential window of 0.5 V, surpassing all the precursors. Electrochemical impedance spectroscopy (EIS) was measured to figure out the charge transfer and ion diffusion mechanism of the samples in the frequency range from 100 kHz to 0.01 Hz. The curve in Figure 4C consists of three regions. The charge transfer resistance (Rct) is determined by the diameter of the semicircle in the high-frequency region, while the diffusion resistance (Rw) of ions can be obtained from the linear slope of the straight line in the low-frequency region. As for the intercept of the curve in the high-frequency region, it means the series resistance (Rs) of the inductive element[44,45]. Benefiting from the excellent conductivity of metal nitrides and nitrogen-doped carbon, the curve of Ni3N-Co2N0.67/NC owns the largest slope in the high-frequency region. Figure 4D shows the CV curve of Ni3N-Co2N0.67/NC at different sweep rates. The curves almost maintain the initial shape with the scan rate increasing from
Figure 4. (A) CV curves, (B) GCD profiles and (C) Nyquist plots in the frequency range from 100 kHz to 0.01 Hz of Ni3N-Co2N0.67/NC, N0C3-TEOA, N1C2-TEOA, N1C1-TEOA, N2C1-TEOA, N3C0-TEOA. (D) CV curves at different scan rates and (E) GCD profiles at different current densities of Ni3N-Co2N0.67/NC. (F) Specific capacitance of Ni3N-Co2N0.67/NC calculated from GCD profiles at different current densities. (G) The cycle performance curve of Ni3N-Co2N0.67/NC at 10 A g-1. (H) b values simulated from anodic and cathodic peak current and scan rate of Ni3N-Co2N0.67/NC. (I) Contribution ratio between capacitance and the diffusion-controlled process under various scan rates.
where i and v represent peak current and scan rate, respectively. a and b are constants. The b value can be calculated through the slope of log(i) versus log(v). A b value of 0.5 means the process is a diffusion-controlled process, while a b value of 1 demonstrates a surface-controlled process[49]. The b value of
where i, v, k1 and k2 are current, scan rate and determined parameters. The capacitive contribution area (green) inside CV curves at different scan rates for Ni3N-Co2N0.67/NC are presented in
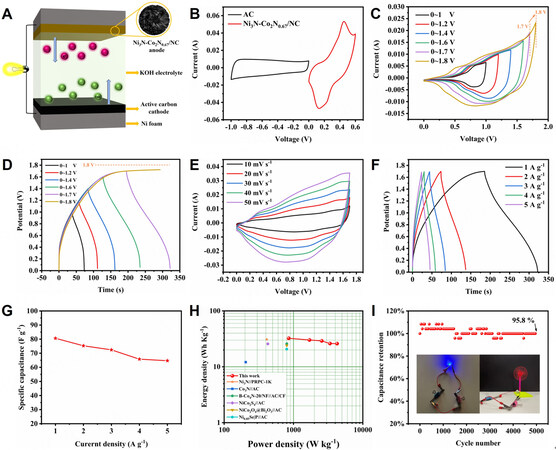
To explore the practical application of Ni3N-Co2N0.67/NC electrode, an asymmetric device was configured applying Ni3N-Co2N0.67/NC and active carbon as positive and negative electrodes in 1 M KOH, respectively. The scheme of the Ni3N-Co2N0.67/NC//AC asymmetric supercapacitor (ASC) is shown in Figure 5A. CV curves and GCD profiles of AC were measured in Supplementary Figure 7A and B to determine the working potential window and mass ratio of positive electrode to negative electrode of the device. The mass ratio of Ni3N-Co2N0.67/NC to AC was calculated to be 0.18 and the working potential widow was estimated to be 1.6 V based on the CV curve of Ni3N-Co2N0.67/NC and AC at 20 mV s-1 [Figure 5B]. To further define the operating potential window, CV curves at 20 mV s-1 and GCD profiles at 1 A g-1 at different operating voltages were performed [Figure 5C and D]. The CV curve met a significant polarization when the operating voltage increased from 1.7 V to 1.8 V and the GCD profile could only reach 1.7 V, so 1.7 V was finally selected as the practical working potential window. CV curves of the ACS at different sweep rates display a pair of redox peaks and remain stable, suggesting Faraday redox characteristics and superior rate capability [Figure 5E]. Calculated from GCD profiles in Figure 5F, the device demonstrates specific capacitance of 80.6, 75.3, 72.4, 65.8 and 64.7 F g-1 at 1, 2, 3, 4 and 5 A g-1 [Figure 5G]. The capacitance remains 80.3% of its initial value when the current density reaches 5 A g-1, indicating excellent capacitive performance. The ASC device delivers a maximum energy density of 32.4 W h Kg-1 at a power density of 851.3 W Kg-1. Even at the maximum power density of 4254.5 W Kg-1, the energy density is still 26 W h Kg-1, surpassing other similar devices reported before such as Ni3N//PRPC-1k[52], Co3N//AC[53],
Figure 5. (A) Scheme of Ni3N-Co2N0.67/NC//AC asymmetric supercapacitor. (B) CV curves of Ni3N-Co2N0.67/NC and AC at 20 mV s-1. (C) CV curves of Ni3N-Co2N0.67/NC//AC ASC at different voltage ranges at 20 mV s-1. (D) GCD profiles of Ni3N-Co2N0.67/NC//AC ASC at different voltage ranges at 1 A g-1. (E) CV curves of Ni3N-Co2N0.67/NC//AC ASC at different scan rates. (F) GCD profiles of
CONCLUSIONS
In conclusion, hollow Ni3N-Co2N0.67/NC nanoflower was synthesized by pyrolyzing nickel/cobalt-TEOA complex precursor using urea as the nitrogen source and applied as positive electrode material for supercapacitor. Thanks to the remarkable electronic conductivity originating from metal nitrides and
DECLARATIONS
Authors’ contributionsDesigned the study and supervised the overall project: Zhu M
Performed the experiments, collected data, and drafted the manuscript: Luo Q
Contributed to the result discussion: Luo Q, Lu C, Liu L, Zhu M
Availability of data and materialsNot applicable.
Financial support and sponsorshipThe authors acknowledge the financial support from the Nation Natural Science Foundation of China (21403091).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
Supplementary MaterialsREFERENCES
1. Wu S, Chen Y, Jiao T, et al. An aqueous Zn-Ion hybrid supercapacitor with high energy density and ultrastability up to 80,000 cycles. Adv Energy Mater 2019;9:1902915.
2. Yu Z, Duong B, Abbitt D, Thomas J. Highly ordered MnO2 nanopillars for enhanced supercapacitor performance. Adv Mater 2013;25:3302-6.
3. Ji J, Zhang LL, Ji H, et al. Nanoporous Ni(OH)2 thin film on 3D Ultrathin-graphite foam for asymmetric supercapacitor. ACS Nano 2013;7:6237-43.
4. Dubal DP, Ayyad O, Ruiz V, Gómez-Romero P. Hybrid energy storage: the merging of battery and supercapacitor chemistries. Chem Soc Rev 2015;44:1777-90.
5. Xu B, Zhang H, Mei H, Sun D. Recent progress in metal-organic framework-based supercapacitor electrode materials. Coord Chem Rev 2020;420:213438.
6. Ma R, Chen Z, Zhao D, et al. Ti3C2Tx MXene for electrode materials of supercapacitors. J Mater Chem A 2021;9:11501-29.
7. Li K, Zhao B, Zhang H, et al. 3D porous honeycomb-like CoN-Ni3N/N-C nanosheets integrated electrode for high-energy-density flexible supercapacitor. Adv Funct Mater 2021;31:2103073.
8. Meng L, Bi J, Gao X, et al. Heterostructure Co2N-Ni3N/NF nanoarrays synthesized by in situ nitriding treatment for high-performance supercapacitor. J Alloys Compd 2022;909:164721.
9. Zhu C, Sun Y, Chao D, et al. A 2.0 V capacitive device derived from shape-preserved metal nitride nanorods. Nano Energy 2016;26:1-6.
10. Xiao X, Peng X, Jin H, et al. Freestanding mesoporous VN/CNT hybrid electrodes for flexible all-solid-state supercapacitors. Adv Mater 2013;25:5091-7.
11. Xiao X, Yu H, Jin H, et al. Salt-templated synthesis of 2D metallic mon and other nitrides. ACS Nano 2017;11:2180-6.
12. Wang H, Li J, Li K, et al. Transition metal nitrides for electrochemical energy applications. Chem Soc Rev 2021;50:1354-90.
13. Yang H, Ning P, Cao H, et al. Selectively anchored vanadate host for self-boosting catalytic synthesis of ultra-fine vanadium nitride/nitrogen-doped hierarchical carbon hybrids as superior electrode materials. Electrochim Acta 2020;332:135387.
14. Jin T, Sang X, Unocic RR, et al. Mechanochemical-assisted synthesis of high-entropy metal nitride via a soft urea strategy. Adv Mater 2018;30:e1707512.
15. Patra S, Roy E, Madhuri R, Sharma PK. Agar based bimetallic nanoparticles as high-performance renewable adsorbent for removal and degradation of cationic organic dyes. J Ind Eng Chem 2016;33:226-38.
16. Patra S, Roy E, Madhuri R, Sharma PK. Nanocomposite of bimetallic nanodendrite and reduced graphene oxide as a novel platform for molecular imprinting technology. Anal Chim Acta 2016;918:77-88.
17. Aziz ST, Kumar S, Riyajuddin S, Ghosh K, Nessim GD, Dubal DP. Bimetallic phosphides for hybrid supercapacitors. J Phys Chem Lett 2021;12:5138-49.
18. Ren F, Ji Y, Chen F, Qian Y, Tian J, Wang J. Flower-like bimetal Ni/Co-based metal-organic-framework materials with adjustable components toward high performance solid-state supercapacitors. Mater Chem Front 2021;5:7333-42.
19. Huang K, Sun Y, Zhang Y, Wang X, Zhang W, Feng S. Hollow-structured metal oxides as oxygen-related catalysts. Adv Mater 2019;31:e1801430.
20. Zhu M, Cheng Y, Luo Q, El-khateeb M, Zhang Q. A review of synthetic approaches to hollow nanostructures. Mater Chem Front 2021;5:2552-87.
21. Liu X, Deng S, Xiao D, et al. Hierarchical bimetallic Ni-Co-P microflowers with ultrathin nanosheet arrays for efficient hydrogen evolution reaction over all pH values. ACS Appl Mater Interfaces 2019;11:42233-42.
22. Dong J, Lu G, Yue J, Cheng Z, Kang X. Valence modulation in hollow carbon nanosphere/manganese oxide composite for high performance supercapacitor. Appl Surf Sci 2019;480:1116-25.
23. Yang Y, Shao Z. Boron and nitrogen co-doped carbon nanospheres for supercapacitor electrode with excellent specific capacitance. Nanotechnology 2022;33:185403.
24. Yang M, Ning H, Xiao L, Cui F, Zhang F. Mn3O4/MnS heterostructure for electrode and asymmetric supercapacitor under high charge/discharge current. Electrochim Acta 2022;424:140630.
25. Qiu L, Yang W, Zhao Q, et al. NiS nanoflake-coated carbon nanofiber electrodes for supercapacitors. ACS Appl Nano Mater 2022;5:6192-200.
26. Ran F, Yang X, Xu X, Li S, Liu Y, Shao L. Green activation of sustainable resources to synthesize nitrogen-doped oxygen-riched porous carbon nanosheets towards high-performance supercapacitor. Chem Eng J 2021;412:128673.
27. Liang J, Li M, Chai Y, Luo M, Li L. TEOA-mediated formation of hollow core-shell structured CoNi2S4 nanospheres as a high-performance electrode material for supercapacitors. J Power Sources 2017;362:123-30.
28. Chen Y, Yang D, Xin X, et al. Multi-stepwise charge transfer via MOF@MOF/TiO2 dual-heterojunction photocatalysts towards hydrogen evolution. J Mater Chem A 2022;10:9717-25.
29. Zhang H, Yao Z, Lan D, Liu Y, Ma L, Cui J. N-doped carbon/V2O3 microfibers as high-rate and ultralong-life cathode for rechargeable aqueous zinc-ion batteries. J Alloys Compd 2021;861:158560.
30. Rezaei B, Hansen TW, Keller SS. Stereolithography-derived three-dimensional pyrolytic carbon/Mn3O4 nanostructures for free-standing hybrid supercapacitor electrodes. ACS Appl Nano Mater 2022;5:1808-19.
31. Xing M, Gao A, Liang Y, et al. Defect-engineered 3D cross-network Co3O4-xNx nanostructure for high-performance solid-state asymmetric supercapacitors. ACS Appl Energy Mater 2021;4:888-98.
32. Rabani I, Zafar R, Subalakshmi K, Kim HS, Bathula C, Seo YS. A facile mechanochemical preparation of Co3O4@g-C3N4 for application in supercapacitors and degradation of pollutants in water. J Hazard Mater 2021;407:124360.
33. Vanaraj R, Vinodh R, Periyasamy T, et al. Capacitance enhancement of metal-organic framework (MOF) materials by their morphology and structural formation. Energy Fuels 2022;36:4978-91.
34. Chen L, Huang Z, Liang H, Gao H, Yu S. Three-dimensional heteroatom-doped carbon nanofiber networks derived from bacterial cellulose for supercapacitors. Adv Funct Mater 2014;24:5104-11.
35. Dong Z, Zhang W, Xiao Y, et al. One-pot-synthesized CoFe-glycerate hollow spheres with rich oxyhydroxides for efficient oxygen evolution reaction. ACS Sustain Chem Eng 2020;8:5464-77.
36. Zhao J, Zou XX, Su J, Wang PP, Zhou LJ, Li GD. Synthesis and photocatalytic activity of porous anatase TiO2 microspheres composed of {010}-faceted nanobelts. Dalton Trans 2013;42:4365-8.
37. Hua W, Sun H, Liu H, Li Y, Wang J. Interface engineered NiMoN/Ni3N heterostructures for enhanced alkaline hydrogen evolution reaction. Appl Surf Sci 2021;540:148407.
38. Hu Y, Luo Z, Guo M, et al. Interface engineering of Co2N0.67/CoMoO4 heterostructure nanosheets as a highly active electrocatalyst for overall water splitting and Zn-H2O cell. Chem Eng J 2022;435:134795.
39. Sun J, Lu J, Huang C, et al. Modification of Ni3N with a cobalt-doped carbon shell for high-performance hydrogen evolution in alkaline media. ACS Sustain Chem Eng 2021;9:1994-2002.
40. Tong R, Xu M, Huang H, et al. Co2N0.67/MoO2 heterostructure as high-efficiency electrocatalysts for the hydrogen evolution reaction. ACS Appl Energy Mater 2022;5:440-8.
41. Wang M, Ma W, Lv Z, Liu D, Jian K, Dang J. Co-doped Ni3N nanosheets with electron redistribution as bifunctional electrocatalysts for efficient water splitting. J Phys Chem Lett 2021;12:1581-7.
42. Inagaki M, Toyoda M, Soneda Y, Morishita T. Nitrogen-doped carbon materials. Carbon 2018;132:104-40.
43. Shi B, Li L, Chen A, Jen TC, Liu X, Shen G. Continuous fabrication of Ti3C2Tx MXene-based braided coaxial Zinc-Ion hybrid supercapacitors with improved performance. Nanomicro Lett 2021;14:34.
44. Ma J, Xia J, Liang Z, Chen X, Du Y, Yan CH. Layered double hydroxide hollowcages with adjustable layer spacing for high performance hybrid supercapacitor. Small 2021;17:e2104423.
45. Wang J, Huang Y, Han X, Li Z, Zhang S, Zong M. A flexible Zinc-Ion hybrid supercapacitor constructed by porous carbon with controllable structure. Appl Surf Sci 2022;579:152247.
46. Peçenek H, Dokan FK, Onses MS, Yılmaz E, Sahmetlioglu E. Outstanding supercapacitor performance with intertwined flower-like NiO/MnO2/CNT electrodes. Mater Res Bull 2022;149:111745.
47. Wang H, Liang M, Duan D, Shi W, Song Y, Sun Z. Rose-like Ni3S4 as battery-type electrode for hybrid supercapacitor with excellent charge storage performance. Chem Eng J 2018;350:523-33.
48. Houpt D, Ji J, Yang D, Choi JH. High-performance supercapacitor electrodes based on composites of MoS2 nanosheets, carbon nanotubes, and ZIF-8 metal-organic framework nanoparticles. ACS Appl Nano Mater 2022;5:1491-9.
49. Yesuraj J, Vajravijayan S, Yang R, et al. Self-assembly of hausmannite Mn3O4 triangular structures on cocosin protein scaffolds for high energy density symmetric supercapacitor application. Langmuir 2022;38:2928-41.
50. Li H, Liu T, He Y, et al. Interfacial engineering and a low-crystalline strategy for high-performance supercapacitor negative electrodes: Fe2P2O7 nanoplates anchored on N/P co-doped graphene nanotubes. ACS Appl Mater Interfaces 2022;14:3363-73.
51. Kim SJ, Sharma V, Kshetri T, Kim NH, Lee JH. Freestanding binder-free electrodes with nanodisk-needle-like MnCuCo-LTH and Mn1Fe2S2 porous microthorns for high-performance quasi-solid-state supercapacitors. ACS Appl Mater Interfaces 2022;14:12523-37.
52. Peng H, Dai X, Sun K, et al. A high-performance asymmetric supercapacitor designed with a three-dimensional interconnected porous carbon framework and sphere-like nickel nitride nanosheets. New J Chem 2019;43:12623-9.
53. Gao J, Zhang W, Zhao Z, Kong L. Solid-phase synthesis and electrochemical pseudo-capacitance of nitrogen-atom interstitial compound Co3N. Sustain Energy Fuels 2018;2:1178-88.
54. Wang Z, Qu G, Wang C, et al. Modified Co4N by B-doping for high-performance hybrid supercapacitors. Nanoscale 2020;12:18400-8.
55. Wang M, An L, Wu M, et al. Self-template synthesis of nickel cobalt sulfide hollow nanotubes for high-performance battery-type supercapacitors. J Electrochem Soc 2021;168:060510.
56. Yu Z, Wang S, Huang Y, et al. Bi2O3 nanosheet-coated NiCo2O4 nanoneedle arrays for high-performance supercapacitor electrodes. J Energy Storage 2022;55:105486.
57. Zhang Y, Wang T, Wang Y, et al. Metal organic frameworks derived hierarchical hollow Ni0.85Se|P composites for high-performance hybrid supercapacitor and efficient hydrogen evolution. Electrochim Acta 2019;303:94-104.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Luo Q, Lu C, Liu L, Zhu M. Triethanolamine assisted synthesis of bimetallic nickel cobalt nitride/nitrogen-doped carbon hollow nanoflowers for supercapacitor. Microstructures 2023;3:2023011. http://dx.doi.org/10.20517/microstructures.2022.41
AMA Style
Luo Q, Lu C, Liu L, Zhu M. Triethanolamine assisted synthesis of bimetallic nickel cobalt nitride/nitrogen-doped carbon hollow nanoflowers for supercapacitor. Microstructures. 2023; 3(2): 2023011. http://dx.doi.org/10.20517/microstructures.2022.41
Chicago/Turabian Style
Luo, Qiao, Congcong Lu, Lingran Liu, Maiyong Zhu. 2023. "Triethanolamine assisted synthesis of bimetallic nickel cobalt nitride/nitrogen-doped carbon hollow nanoflowers for supercapacitor" Microstructures. 3, no.2: 2023011. http://dx.doi.org/10.20517/microstructures.2022.41
ACS Style
Luo, Q.; Lu C.; Liu L.; Zhu M. Triethanolamine assisted synthesis of bimetallic nickel cobalt nitride/nitrogen-doped carbon hollow nanoflowers for supercapacitor. Microstructures. 2023, 3, 2023011. http://dx.doi.org/10.20517/microstructures.2022.41
About This Article
Copyright
Data & Comments
Data
 Cite This Article 14 clicks
Cite This Article 14 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.